In this issue:
This study investigates the extent to which a windscreen affects the severity of laser eye dazzle (disability glare produced by a laser) experienced by a human observer. Windscreen scatter measurements were taken for a range of windscreens in a variety of conditions, showing that windscreen scatter is similar in magnitude to scatter from the human eye. Human subject experiments verified that obscuration angles caused by laser eye dazzle could be increased by the presence of a windscreen when comparing a dirty automobile windscreen to an eye-only condition with a 532-nm laser exposure. However, a light aircraft windscreen with lower scatter did not exhibit increased obscuration angles at 532 nm, and neither windscreen exhibited an increase at 635 nm. A theoretical analysis of laser eye dazzle, using measured windscreen scatter functions, has provided insight into the delicate interplay between scatter, transmission and the angular extent of dazzle. A model based on this analysis has been shown to be a useful tool to predict the impact of windscreens on laser eye dazzle, with the goal of informing future updates to the authors’ laser eye dazzle safety framework.
Implementation
The experiment was conducted indoors using two commercially available laser sources: a green continuous wave
532-nm laser and a red continuous wave
635-nm laser Collectively, green and red accounted for 93% of all laser illuminations reported to the FAA in 2017 [1], which demonstrates the relevance of this laser choice.
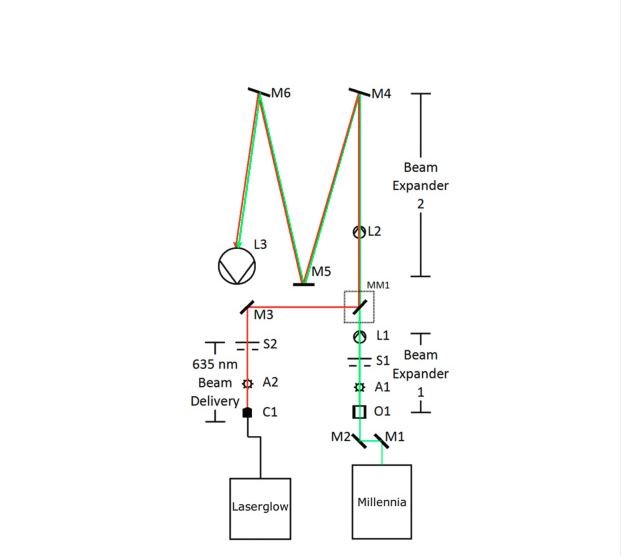
A schematic diagram of the optical arrangement is shown in Fig. 1 and a list of the primary optical components is given in Table 1. Both laser beams passed through a series of optics to provide wide, collimated beams. The 532-nm laser beam was first expanded 12 × to provide a 28 mm beam that more closely matched the 27 mm output from the fiber collimator of the 635-nm laser. A second beam expander then provided an additional 12 × expansion of both laser sources, after which the beam was directed onto a 500 mm diameter spherical collimating mirror with a focal length, f, of 4000 mm. The final collimated output beam, which was 100 mm in diameter, was reflected off a plano turning mirror before it was incident on the front surface of the windscreen.
Conclusion
This present work supplements previous studies by combining scatter measurements, human subject experiments and a theoretical treatment, while exploring a much wider range of parameters in order to develop a deeper understanding of how windscreen scatter impacts laser eye dazzle. For the first time, scatter dependencies on windscreen type, surface condition, incidence angle, and laser wavelength are all explored within the same experiment. Furthermore, human visual performance in the presence of laser eye dazzle is then assessed, both with and without windscreens in place, at laser irradiances up to 600 µW·cm-2 at the eye. Finally, a theoretical analysis brings together the experimental data with a state-of-the-art dazzle prediction methodology.
Full access to the materials and methodology, can be found by
clicking here.
To see the 635-nm laser
click here, to see the 532-nm laser
click here


