In this issue:
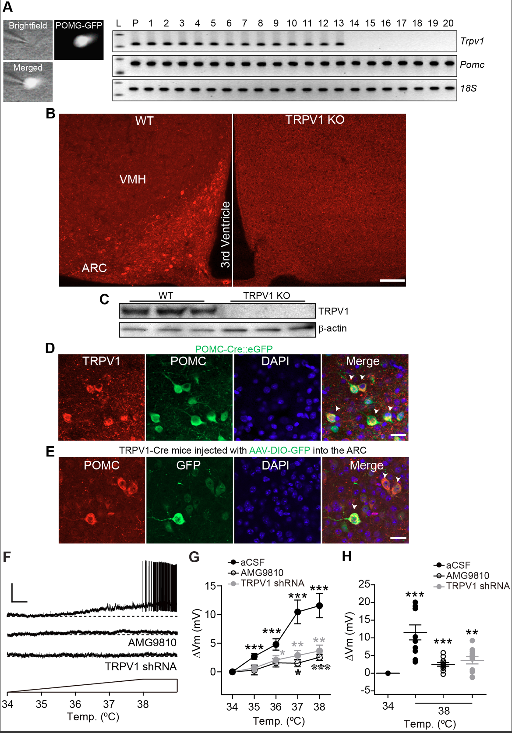
Proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARC) respond to numerous hormonal and neural signals, resulting in changes in food intake. Here, they demonstrate that ARC POMC neurons express capsaicin-sensitive transient receptor potential vanilloid 1 receptor (TRPV1)-like receptors. To show expression of TRPV1-like receptors in ARC POMC neurons, they use single-cell reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry, electrophysiology, TRPV1 knock-out (KO), and TRPV1-Cre knock-in mice. A small elevation of temperature in the physiological range is enough to depolarize ARC POMC neurons. This depolarization is blocked by the TRPV1 receptor antagonist and by Trpv1 gene knockdown. Capsaicin-induced activation reduces food intake that is abolished by a melanocortin receptor antagonist. To selectively stimulate TRPV1-like receptor-expressing ARC POMC neurons in the ARC, they generate an adeno-associated virus serotype 5 (AAV5) carrying a Cre-dependent channelrhodopsin-2 (ChR2)–enhanced yellow fluorescent protein (eYFP) expression cassette under the control of the two neuronal POMC enhancers (nPEs). Optogenetic stimulation of TRPV1-like receptor-expressing POMC neurons decreases food intake. Hypothalamic temperature is rapidly elevated and reaches to approximately 39 °C during treadmill running. This elevation is associated with a reduction in food intake. Knockdown of the Trpv1 gene exclusively in ARC POMC neurons blocks the feeding inhibition produced by increased hypothalamic temperature. Taken together, the findings identify a melanocortinergic circuit that links acute elevations in hypothalamic temperature with acute reductions in food intake.
Material & Methods
Mice were maintained under isoflurane anesthesia and placed in a stereotaxic apparatus. Under aseptic conditions, sterile custom guide cannulas were stereotaxically implanted into the ARC (AP, −1.2 mm; ML, 0; DV, −5.5 mm) for local injection of drugs (1 μL of 100 nM capsaicin, Sigma-Aldrich, cat # M2028 and AMG9810, Tocris bioscience, cat # 2316) and into the lateral ventricle (AP, +0.85 mm; ML, +0.5 mm; DV, −3.0 mm) for injection of SHU9119 (Tocris bioscience, cat # 3420) or naloxone (Tocris bioscience, cat # 0599). Animals were allowed at least a week to recover from surgery. To measure food intake, animals were separated individually in single cages for 5 d and fasted for 6 h (from 1 PM to 7 PM) before drug infusion. SHU9119 (1 μL of 5 μM) or naloxone (1 μL of 1 mM) was intracerebroventricularly administrated 30 min prior to microinjection of capsaicin or saline. Capsaicin and/or AMG9810 were directly infused into the ARC at 6 PM. Food intake was measured from 7 PM to 11 PM and from 11 PM to 7 AM. The mice used for all of the experiments were randomly assigned to a treatment group. For animal studies, sample sizes were estimated according to the previous experience. Single housed mice with a 20% weight loss were excluded from experiments and analyses. For optogenetic experiments, a mono fiber-optic cannula of 230 μm diameter was implanted into the ARC and coupled to our 473-nm DPSS laser.
Conclusion
Mice were maintained under isoflurane anesthesia and placed in a stereotaxic apparatus. Under aseptic conditions, sterile custom guide cannulas were stereotaxically implanted into the ARC (AP, −1.2 mm; ML, 0; DV, −5.5 mm) for local injection of drugs (1 μL of 100 nM capsaicin, Sigma-Aldrich, cat # M2028 and AMG9810, Tocris bioscience, cat # 2316) and into the lateral ventricle (AP, +0.85 mm; ML, +0.5 mm; DV, −3.0 mm) for injection of SHU9119 (Tocris bioscience, cat # 3420) or naloxone (Tocris bioscience, cat # 0599). Animals were allowed at least a week to recover from surgery. To measure food intake, animals were separated individually in single cages for 5 d and fasted for 6 h (from 1 PM to 7 PM) before drug infusion. SHU9119 (1 μL of 5 μM) or naloxone (1 μL of 1 mM) was intracerebroventricularly administrated 30 min prior to microinjection of capsaicin or saline. Capsaicin and/or AMG9810 were directly infused into the ARC at 6 PM. Food intake was measured from 7 PM to 11 PM and from 11 PM to 7 AM. The mice used for all of the experiments were randomly assigned to a treatment group. For animal studies, sample sizes were estimated according to the previous experience. Single housed mice with a 20% weight loss were excluded from experiments and analyses. For optogenetic experiments, a mono fiber-optic cannula of 230 μm diameter was implanted into the ARC and coupled to our 473-nm DPSS laser.
Conclusion
In summary, the team identify a melanocortinergic circuit beginning in ARC POMC neurons that links acute elevations in peripheral and brain temperature with acute reductions in food intake via activation of TRPV1-like receptors. Such temperature-sensing mechanisms are ideally situated to mediate the regulation of energy intake and expenditure.
Full access to the materials and methodology, can be found by
clicking here.
Details on the 473 nm NIR Laser used in the research can be found by
clicking here.